All Publications
Please select the period from the menubar on the left or follow the links below.
 |
Exploring the Mechanism of Phase Transitions between the Hexagonal Close-Packed and the Cuboidal Structures:
The cover depicts the Burgers-Bain path on a cohesive energy hypersurface for the hcp-to-bcc transition of a Lennard-Jones system demonstrating that the fcc structure is an intermediate in the phase transition. Our paper shows that a very similar topology for the energy hypersurface is obtained from density functional calculations of metallic lithium.
|
 |
From Hard to Soft Spheres in Barlow Packings:
Barlow packings are the densest possible packings characterized by configurations combining
hexagonal close packing (hcp) and face-centered cubic (fcc) sequences, and there are an infinite
number of variations. It is surprising that such packings, like the 9R structure, are rarely
observed in nature. This work presents an analysis of periodic Barlow packings, showing that
the cohesive energies for hcp and fcc for Lennard–Jones systems are extremal, positioning
Barlow packings energetically between hcp and fcc configurations.
|
 |
Exploring the limit of the periodic table:
The cover of this issue illustrates the search for an island of nuclear stability, a metaphor that has by now shifted towards glimpsing the mountains of enhanced stability on the horizon, their tops still concealed by clouds. See Smits et al, Nature Review Physics (2024). Cover design by Susanne Harris.
|
 |
The Lennard-Jones potential Revisited: The Lennard-Jones potential can be extended to an inverse power series, resulting in simple lattice sum expressions for the equations of state of a solid, including harmonic and anharmonic vibrational effects. This is demonstrated for the rare gas elements from helium down to oganesson in the periodic table.
|
 |
Superheavy element chemistry: Exploring the chemical nature of super-heavy main-group elements by means of efficient plane-wave density-functional theory.
|
 |
Oganesson, a relativistic element: Three decay chains within the millisecond range were observed for the heaviest element in the periodic table, the 294 isotope of oganesson. This currently precludes any chemical investigation, but highly accurate coupled cluster calculations for the solid state reveal the unusual physical behavior of oganesson due to spin-orbit coupling in the 7p shell.
|
 |
Change it up: A state-of-the-art quantum theoretical treatment reveals a detailed picture of the photochemical isomerization process from the nitrate to the cis or trans peroxynitrite. The influence of the aqueous solution is also considered.
|
 |
Electron and Nucleon Localization Functions of Oganesson: Approaching the Thomas-Fermi Limit. Calculations of the structure in oganesson, the element with the highest atomic number, reveal a uniform, gas-like distribution of its electrons and nucleons.
|
 |
The attachment of a suitable "bouncer" molecule to the rim of a graphene pore
prevents the passage of the undesired enantiomer while
letting its mirror image through. A small difference in the geometry of the
temporary dimer complex, which is formed by the "bouncer" and the penetrating
molecule, is transformed into a significant difference for the transmission barrier.
For more information see A. W. Hauser, N. Mardirossian, J. A. Panetier,
M. Head-Gordon, A. T. Bell, P. Schwerdtfeger, Angew. Chem. Int. Ed. 53, 9957 (2014).
|
 |
The cover picture of J. Chem. Inf. Mod. shows T-C380, the first fullerene that does not admit any face spirals, partially
assembled until the point where the spiral fails. Below the fullerene, a schematic drawing
of the partially spiraled fullerene graph is shown. The T-C380 molecule was generated
using the computer program Fullerene using a generalized spiral algorithm discussed in
the paper J. Chem. Inf. Mod. 54, 121 (2014).
|
 |
The cover picture of our Angewandte Chemie paper (2013) (designed by Cameron Smorenburg)
shows the link between Albert Einstein's special theory of relativity and the fact that mercury
is liquid at room temperature. An old problem is now solved: Monte Carlo
simulations using the diatomic-in-molecule method derived from accurate
ground- and excited-state relativistic calculations for Hg2 show that the melting
temperature for bulk mercury is lowered by 105 K, which is due to relativistic effects.
For more information see F. Calvo, E. Pahl, M. Wormit, P. Schwerdtfeger,
Angew. Chem. Int. Ed. 52, 7583 (2013).
|
 |
The cover picture of our Angewandte Chemie paper (2010) (designed by Roman Schwerdtfeger)
contains the words of Galadriel to Frodo in
'The Lord of the Rings' by J. R. R. Tolkien and continues: “But the mirror will also show
things unbidden, and those are often stranger and more profitable than things which we wish
to behold. What you will see, if you leave the Mirror free to work, I cannot tell.
For it shows things that were, and things that are, and things that yet may be.
But which it is that he sees, even the wisest cannot always tell.”
The large parity violation effects predicted for the chiral molecule
NWHClI from relativistic density functional theory are shown as a broken mirror image.
The energy difference of 0.7 Hz for the N-W stretching frequency conveniently lies in the
frequency range of CO2 lasers and may be revealed by future high-resolution spectroscopy experiments.
For more information see D. Figgen, A. Koers, P. Schwerdtfeger, Angew. Chem. Int. Ed. 49,
2941 (2010).
|
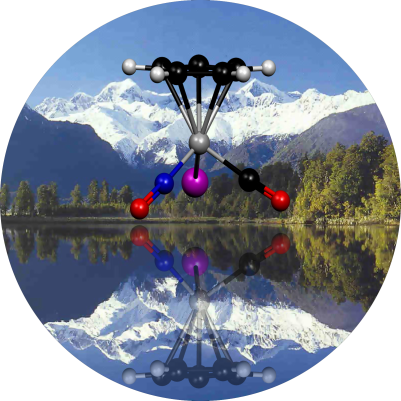
|
The cover picture of Angewandte Chemie (2003) shows Lake Matheson in New
Zealand, famous for its reflections of highest peaks Mount Cook and Mount
Tasman. In the macroscopic world mirror images are not perfect due to
imperfections in the mirror. However, at Lake Matheson the waters are often
incredibly still and it is difficult to tell the mountains from its reflection.
In the microscopic world mirror image symmetry is violated as well (parity
violation) and here it is even more difficult to distinguish between
enantiomers. High resolution spectroscopy gave so far no indication for the
breakdown of mirror image symmetry in molecules. The
(C5H5)Re(CO)(NO)I molecule shown in the cover picture
gives unprecedented large parity violation energy differences of 300 Hz which
brings us a step closer to the detection of such tiny effects. For more information see
P. Schwerdtfeger, J. Gierlich and T. Bollwein, Angew. Chem. Int. Ed.
42, 1293 (2003).
|
|
Selected Publications
A. Robles-Navarro, S. Cooper, A. A. Buchheit, J. Busse, A. Burrows, O. Smits, P. Schwerdtfeger, "Exact lattice summations for Lennard-Jones potentials coupled to a three-body Axilrod-Teller-Muto term applied to cuboidal phase transitions" J. Chem. Phys.163, 094104-1-35 (2025).
S. Cooper, A. Robles-Navarro, O. R. Smits, P. Schwerdtfeger, "The Theory of Barlow Packings: Basic Properties and Lattice Sums for Inverse Power Potentials", J. Chem. Phys. 162, 014105-1-22 (2025).
S. Cooper, A. Robles-Navarro, O. R. Smits, P. Schwerdtfeger, "From Hard to Soft Dense Sphere Packings: The Cohesive Energy of Barlow Structures Using Exact Lattice Summations for a General Lennard-Jones Potential", J. Chem. Phys. Lett. 15, 8387-8392 (2024).
P. Schwerdtfeger, D. J. Wales, "100 Years of the Lennard-Jones Potential", J. Chem. Theory Comput. 20, 3379-3405 (2024).
O. R. Smits, Ch. Düllmann, P. Indelicato, W. Nazarewicz, P. Schwerdtfeger, "The quest for superheavy elements: pushing the Periodic Table to the limit", Nature Rev. Phys. 6, 86-98 (2024).
A. Robles-Navarro, P. Jerabek, P. Schwerdtfeger, "Tipping the balance between the bcc and fcc phase within the alkali and coinage metal groups", Angew. Chem. Int. Ed. 63, e202313679 (2024).
O. R. Smits, P. Indelicato, W. Nazarewicz, M. Piibeleht, P. Schwerdtfeger, "Pushing the Limits of the Periodic Table - A Review on Atomic Relativistic Electronic Structure Theory and Calculations for the Superheavy Elements", Phys. Rep. 1035, 1-57 (2023).
S. Löffelsender, P. Schwerdtfeger, S. Grimme, J.-M. Mewes, "It's Complicated: On Relativistic Effects and Periodic Trends in the Melting and Boiling Points of the Group 11 Coinage Metals", J. Am. Chem. Soc. 144, 495-502 (2022).
J.-M. Mewes, P. Schwerdtfeger, "Exclusively Relativistic: Periodic Trends in the Melting and Boiling Points of Group 12", Angew. Chem. Int. Ed. 60, 7703-7709 (2021).
O. Smits, J.-M. Mewes, P. Jerabek, P. Schwerdtfeger, "Oganesson - A noble gas element that is neither noble nor a gas", Angew. Chem. Int. Ed. 59, 23636-23640 (2020).
P. Schwerdtfeger, O. Smits, P. Pyykkö, "The Periodic Table and the Physics That Drives It", Nature Rev. Chem. 4, 359-380 (2020).
A. Burrows, S. Cooper, E. Pahl, P. Schwerdtfeger, "Analytical methods for fast converging lattice sums for cubic and hexagonal close-packed lattices" J. Math. Phys. 61, 123503-1-35 (2020).
J.-M. Mewes, O. R. Smits, G. Kresse, P. Schwerdtfeger, "Copernicium is a Relativistic Noble Liquid", Angew. Chem. Int. Ed. 58, 17964-17968 (2019).
J.-M. Mewes, P. Jerabek, O. R. Smits, P. Schwerdtfeger, "Oganesson is a Semiconductor: Relativistic Band-Gap Narrowing in the Heaviest Rare-Gas Solids", Angew. Chem. Int. Ed. 58, 14260-14264 (2019). (selected as hot paper)
S. A. Giuliani, Z. Matheson, W. Nazarewicz, E. Olsen, P.-G. Reinhard, J. Sadhukhan, B. Schuetrumpf, N. Schunck, P. Schwerdtfeger, "Oganesson and beyond", Rev. Mod. Phys. 91, 011001-1-25 (2019).
L. F. Pasteka, Y. Hao, A. Borschevsky, V. V. Flambaum, P. Schwerdtfeger, "Material Size Dependence on Fundamental Constants", Phys. Rev. Lett. 122, 160801-1-8 (2019). (highlighted and featured in Physics)
J.-M. Mewes, P. Jerabek, D. S. Bohle, P. Schwerdtfeger, "On the Light-Driven Isomerization of
Aqueous Nitrate: A Theoretical Perspective", ChemPhotoChem 2, 725-733 (2018). (highlighted in ChemPhotoChem)
O. Smits, P. Jerabek, E. Pahl, P. Schwerdtfeger, "A Hundred-Year-Old Experiment Re-evaluated: Accurate Ab-Initio Monte-Carlo Simulations of the Melting of Radon",
Angew. Chem. Int. Ed. 57, 9961-9964 (2018).
P. Jerabek, B. Schuetrumpf, P. Schwerdtfeger, W. Nazarewicz, "Electron and Nucleon Localization Functions
in Superheavy Elements", Phys. Rev. Lett. 120, 053001-1-5 (2018). (Editor's choice, highlighted in APS Physics and cover page).
L. F. Pasteka, E. Eliav, A. Borschevsky, U. Kaldor, and P. Schwerdtfeger, "Relativistic coupled cluster calculations with variational quantum electrodynamics resolve the discrepancy between experiment and theory concerning the electron affinity and ionization potential of gold", Phys. Rev. Lett. 118, 023002-1-5 (2017). (Editor's choice and highlighted in APS Physics).
P. Schwerdtfeger, R. Tonner, G. A. Moyano, E. Pahl, "Towards J/mol Accuracy for the Cohesive Energy of Solid Argon",
Angew. Chem. Int. Ed. 55, 12200-12205 (2016).
L. Trombach, S. Rampino, L.-S. Wang, P. Schwerdtfeger, "Hollow Gold Cages and their Topological Relationship to Dual Fullerenes", Chem. Europ. J. 22, 8823-8834 (2016). (selected as hot paper, dedicated to Prof. G. Frenking on the occasion of his 70th birthday).
P. Schwerdtfeger, L. Wirz, J. Avery, "The Topology of Fullerenes",
WIREs Comput. Mol. Sci. 5, 96-145 (2015).
D. Sundholm, L. N. Wirz, P. Schwerdtfeger, "Novel hollow all-carbon structures",
Nanoscale 7, 15886-15894 (2015).
D. K. Theilacker, B. Schlegel, M. Kaupp, P. Schwerdtfeger, "Relativistic and Solvation
Effects on the Stability of Gold(III) Halides in Aqueous Solution",
Inorg. Chem. 54, 9869-9875 (2015).
A. W. Hauser, N. Mardirossian, J. A. Panetier, M. Head-Gordon, A. T. Bell, P. Schwerdtfeger,
"Functionalized graphene as a gatekeeper for chiral molecules: A new concept for chiral separation",
Angew. Chem. Int. Ed. 53, 9957-9960 (2014).
L. Wirz, R. Tonner, J. Avery, P. Schwerdtfeger, "Structure and Properties of the Non-Spiral Fullerenes
T-C380, D3-C384, D3-C440
and D3-C672 and their Halma and Leapfrog Transforms",
J. Chem. Inf. Mod. 54, 121-130 (2014).
J. Wiebke, P. Schwerdtfeger, E. Pahl, "Melting at High Pressure: Can First-Principles Computational
Chemistry Challenge Diamond-Anvil Cell Experiments?", Angew. Chem. Int. Ed. 52, 13202-13205 (2013).
P. Schwerdtfeger, "One flerovium atom at a time", Nature Chem. 5, 636 (2013).
F. Calvo, E. Pahl, M. Wormit, P. Schwerdtfeger, "Evidence for low temperature melting of mercury owing to
relativity", Angew. Chem. Int. Ed. 52, 7583-7585 (2013).
P. Schwerdtfeger, L. Wirz, J. Avery, "Program Fullerene - A Software Package for Constructing
and Analyzing Structures of Regular Fullerenes", J. Comput. Chem. 34, 1508-1526 (2013).
D. A. Götz, R. Schäfer, P. Schwerdtfeger, "The performance of density functional and
wavefunction based methods for the 2D and 3D structures of Au10", J. Comput. Chem.
34, 1975-1981 (2013).
A. Hauser, P. Schwerdtfeger, "Nanoporous graphene membranes for efficient 3He/4He
separation", J. Phys. Chem. Lett. 3, 311-318 (2012).
K. Beloy, A. W. Hauser, A. Borschevsky, V. V. Flambaum, P. Schwerdtfeger,
"Effect of α -variation on the vibrational spectrum of Sr2",
Phys. Rev. A 84, 062114-1-4 (2011).
A. Hermann, P. Schwerdtfeger, "Opening of the UV-window for water under pressure",
Phys. Rev. Lett. 106, 187403-1-4 (2011).
D. Figgen, A. Koers, P. Schwerdtfeger, "NWHClI - A small and compact chiral molecule
with large parity violation effects in the vibrational spectrum.",
Angew. Chem. Int. Ed. 49, 2941-2943 (2010).
B. Vest, A. Hermann, P. Schwerdtfeger, "The Nucleation of (Anti)ferromagnetically Coupled Chromium
Dihalides - From Small Clusters to the Solid State", Inorg. Chem. 49, 3169-3182 (2010).
C. Thierfelder, P. Schwerdtfeger, A. Koers, A. Borschevsky, B. Fricke, "Scalar relativistic and
spin-orbit effects in closed-shell superheavy element monohydrides", Phys. Rev. A 80,
022501-1-10 (2009).
S. Biering, A. Hermann, J. Furthmüller, P. Schwerdtfeger, "The unusual solid state structure of mercury oxide:
A first principle relativistic density functional study for the group 12 oxides ZnO, CdO and HgO",
J. Phys. Chem. A 113, 12427-12432 (2009).
A. Hermann, P. Schwerdtfeger, "Ground state properties of crystalline ice from periodic Hartree-Fock and a
coupled cluster based many-body decomposition of the correlation energy", Phys. Rev. Lett.
101, 183005-1-4 (2008).
E. Pahl, F. Calvo, L. Koci, P. Schwerdtfeger, "Towards accurate melting temperatures from ab initio
Monte Carlo simulations for neon and argon: from clusters to the bulk",
Angew. Chem. Int. Ed. 47, 8207-8210 (2008).
A. Hermann, W. G. Schmidt, P. Schwerdtfeger, "Resolving the optical spectrum of water:
Coordination and electrostatic effects", Phys. Rev. Lett. 100, 207403-1-4 (2008).
A. Hermann, M. Lein, P. Schwerdtfeger, "The Gregory-Newton Problem of Kissing Sphere Applied to Chemistry:
The Search for the Species with the Highest Coordination Number.",
Angew. Chem. Int. Ed. 46, 2444-2447 (2007).
N. Gaston, I. Opahle, H. W. Gäggeler, P. Schwerdtfeger,
"Is Eka-Mercury (Element 112) a Group 12 Metal",
Angew. Chem. Int. Ed. 46, 1663-1666 (2007).
P. Schwerdtfeger, N. Gaston, R. P. Krawczyk, R. Tonner, G. E.
Moyano, "Theoretical investigations into rare gas clusters and
crystal lattices of He, Ne, Ar and Kr using many-body interaction
expansions - the Lennard-Jones Potential revised", Phys. Rev. B.
73, 064112-1-19 (2006).
N. Gaston, P. Schwerdtfeger, "From the van der Waals dimer to the solid-state of
mercury with relativistic ab initio and density functional theory", Phys. Rev. B
74, 024105-1-12 (2006).
P. Schwerdtfeger, T. Saue, J. N. P. van Stralen, L. Visscher,
"Relativistic Second-Order Many-Body and Density Functional
Theory for the Parity-Violation Contribution to the C-F Stretching
Mode in CHFClBr.", Phys. Rev. A 71,
012103-1-7 (2005).
J. Crassous, Ch. Chardonnet, T. Saue, P. Schwerdtfeger, "Recent
experimental and theoretical developments towards the observation of
parity violation (PV) effects in molecules by spectroscopy", Org.
Biomol. Chem. 3, 2218-2224 (2005).
P. Schwerdtfeger, R. Bast, “Large Parity Violation Effects in the Vibrational Spectrum of
Organometallic Compounds”, J. Am. Chem. Soc. 126, 1652-1653
(2004).
P. Schwerdtfeger, R. P. Krawczyk, A. Hammerl, R. Brown, "A
Comparison of Structure and Stability between the Group 11 Halide
Tetramers M4X4 (Cu, Ag, Au; X= F, Cl, Br and I) and the Group 11
Chloride and Bromide Phosphanes (XMPH3)4", Inorg.
Chem. 43,
6707-6716 (2004).
R. Bast and P. Schwerdtfeger, “Parity Violation Effects in the C-F Stretching Mode of Heavy
Atom Containing Methyl Fluorides”, Phys. Rev. Lett. 91,
023001-1-3 (2003).
P. Schwerdtfeger, “Gold
Goes Nano – From Small Clusters to Low-Dimensional Assemblies.”, Angew.
Chem. Int. Ed. 42, 1892-1895 (2003).
|